Tags
antibiotic resistance, antibiotics, bacteria, bacteriophage, disease, evolution, health, medical treatments, phage therapy, viral therapy, virus

Though I’m no spring chicken, I’m still too young to have experienced the Golden Age of the Antibiotic Era. In those halcyon days, antibiotics were heralded as the “magic bullet” that enabled man to decisively conquer disease; the ultimate symbol of civilization successfully resisting Mother Nature to the betterment of society. Back then, the antibiotic pill was the all powerful, multipurpose solution for whatever microbial illness ailed you. It was easy, convenient, and cost-effective–the perfect medicine for a capitalist society.
A lot of things have undoubtedly changed since then, for better or for worse. The hyperbolic optimism of yesteryear has been replaced with fears of devastating disease outbreaks due to the biological blowback of unintended consequences. Drug resistant bacteria is now officially acknowledged as a serious threat to public health–last month, the Obama Administration announced an ambitious plan to combat the growing problem, which according to the CDC has caused at least 2 million illnesses and 23,000 deaths every year in the U.S. alone.
Ignoring The Lessons Of Evolution For Convenience And Profit
There are many factors that have contributed to this looming public health disaster. According to Forbes, Big Agriculture has unwittingly turbocharged bacterial evolution with its indiscriminate use of herbicides and antibiotics on livestock:
“Herbicides are fairly ubiquitous in the environment. Glyphosate (Roundup) has been found in the milk and meat of cows, and in human urine. According to German researchers, “Glyphosate residues cannot be removed by washing and they are not broken down by cooking. Glyphosate residues can remain stable in foods for a year or more, even if the foods are frozen, dried or processed.” Thus, there is great chance for interaction of herbicides with antibiotics. Interestingly, Roundup alone had once been considered as an antibiotic, but resistance was found to develop rapidly…
In addition, “Antibiotic use has been soaring, with 80% of antibiotics being used in livestock production. In 2010, an estimated 63,151 tons of antibiotics were consumed by livestock; this is projected to increase by 67% by 2030.” Judy Stone, Forbes
However, the medical and pharmaceutical industry are far from blameless. While personal anecdote should never replace hard research, I can attest from personal experience that doctors will often prescribe antibiotics for a cold. Colds are caused by viruses, so drugs that are designed to kill bacteria are obviously useless. Yet this sort of institutional stupidity continues to persist, despite the danger it poses to the population at large:
“A new study out in the Journal of Antimicrobial Chemotherapy found that for some conditions rates of prescription are flying in the face of national best practices, with more antibiotics prescribed for coughs and colds now than before there were any recommendations to reduce antibiotic resistance. In the study, researchers led by Jeremy Hawker of Public Health England looked at data from 537 UK general practice surgeries from 1995 to 2011.
“They found that in 1995, 47 percent of cough and cold episodes were prescribed antibiotics. This went down to 36 percent by 1999 but then raced up to 51 percent in 2011. That’s half of all patients with coughs and colds receiving antibiotics, even though the recommendations tell GPs one of the things they can do is “no prescribing of antibiotics for simple coughs and colds.” Victoria Turk, Motherboard
Meanwhile, the development of new antibiotic drugs have been put on the backburner due to its lack of profitability. According to the International Business Times:
“One reason the number of new antibiotics is so limited is that pharmaceutical companies have little financial incentive to invest in them. Drugs that treat chronic diseases are far more profitable than antibiotics, which are taken for a limited time.”
As Dr. Peg Riley, a leading microbial researcher at the University of Massachusetts Amherst, wryly observed: “[The government] stopped funding antibiotic development, and pharmaceutical companies stopped funding it because they thought they had the battle won…How can you argue to your investors to invest $1 billion in a drug that you hopefully won’t have to use very often?”
The Promise Of Phage Therapy: Infecting The Bacteria That’s Infecting You
Treating a bacterial infection with a virus may seem like a novel idea now, but this old school treatment actually predates the development of the modern antibiotic. Bacteriophages are ubiquitous in the natural environment, and can be found everywhere–in the oceans, in the soil, in our homes, and inside our bodies. We regularly ingest them without any adverse effects. And the best part: it’s the kind of medicine that can evolve in lockstep with the disease it’s targeted to treat.
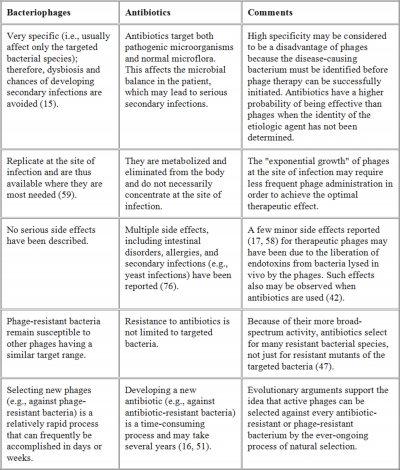
Phage therapy is readily available in Russia and Georgia, though here in the West it has yet to leave the testing phase. Ironically, phage therapy was first discovered and developed by two Western scientists: English bacteriologist Frederick Twort and French Canadian microbiologist Felix D’Herelle. Unfortunately, phage therapy initially proved to be inconsistent due to time constraints; determining the exact cause of the infection and selecting the corresponding phage can be quite time consuming, and sick patients looking for relief may not be in any position to wait. In this case, the specific nature of phage therapy was a major disadvantage for people who needed a quick diagnosis and cure.
The discovery of penicillin in 1941 effectively buried phage therapy in the West, due to its convenient broad spectrum nature. It could kill a wide variety of pathogenic bacteria, which was a major time saver–doctors didn’t have to spend too much time diagnosing, because antibiotics could take care of it regardless of what it actually was. Efficiency eventually lulled the scientific and medical establishment into complacency; it was only a matter of time before the costs of microbial collateral damage would catch up with us.
While the potential for phage therapy looks bright, there are some disadvantages that can inhibit its development, as documented by Wikipedia:
- The high bacterial strain specificity of phage therapy may make it necessary for clinics to make different cocktails for treatment of the same infection or disease because the bacterial components of such diseases may differ from region to region or even person to person.
- In addition, due to the specificity of individual phages, for a high chance of success, a mixture of phages is often applied. This means that ‘banks’ containing many different phages must be kept and regularly updated with new phages.
- Further, bacteria can evolve different receptors either before or during treatment; this can prevent the phages from completely eradicating the bacteria.
- The need for banks of phages makes regulatory testing for safety harder and more expensive. Such a process would make it difficult for large-scale production of phage therapy. Additionally, patent issues (specifically on living organisms) may complicate distribution for pharmaceutical companies wishing to have exclusive rights over their “invention”, making it unlikely that a for-profit corporation will invest capital in the widespread application of this technology.
- To work, the virus has to reach the site of the bacteria, and viruses do not necessarily reach the same places that antibiotics can reach.
- Funding for phage therapy research and clinical trials is generally insufficient and difficult to obtain, since it is a lengthy and complex process to patent bacteriophage products. Scientists comment that ‘the biggest hurdle is regulatory’, whereas an official view is that individual phages would need proof individually because it would be too complicated to do as a combination, with many variables. Due to the specificity of phages, phage therapy would be most effective with a cocktail injection, which is generally rejected by the U.S. Food and Drug Administration (FDA). Researchers and observers predict that for phage therapy to be successful the FDA must change its regulatory stance on combination drug cocktails. Public awareness and education about phage therapy are generally limited to scientific or independent research rather than mainstream media.
Despite the considerable obstacles, it’s clear that there’s no more time to waste when it comes to solving this public health issue. Rapidly evolving pathogens have already outsmarted our best drugs, so this requires the best out of the box thinking our society has to offer. With enough political will and economic investment, phage therapy could very well become the future of medicine, as long as we’re willing to rediscover what we once overlooked.

Interesting point of view on this developing technology. This year is year of phage.
LikeLike